|
|
| Module | CO2 equilibrium concentrations in MAP seafood |
| Reference | Ross, T. and Dalgaard, P. (2004). Secondary models.
In: McKeller, R.C.and Lu, X. (Eds.), Modeling Microbial Responses in
Foods. CRC Press, Boca Raton, USA, pp. 63-150. |
| Factors in model | Temperature, initial gas/product ratio, initial percentage of CO2 |
| Range of applicability | FSSP uses this module for seafood with 0-8% NaCl in the water phase, pH between 5.6 and 7.7 and storage temperatures from 0°C to 25°C. |
| When seafood is packed in modified atmospheres with CO2
this gas dissolves into the water and lipid phases of the products.
CO2 dissolves gradually in chilled fresh fish with 50% of
the equilibrium concentration being dissolved in less than 5 hours and >90% being dissolved
after 24 hours (Sivertsvik et al. 2004; Rotabakk et al. 2008). At equilibrium the relation
between the concentrations of CO2 in the headspace gas phase
and the concentration dissolved in the water/lipid phase of fresh fish is determined by Henry's law (Eqn.
1).
In Eqn. 1 KH is Henry’s constant (mg l-1 atm-1) and pCO2 the partial pressure of CO2 (atm.). Compared to oxygen and nitrogen large amounts of CO2 can be dissolved in fresh fish. Therefore, the gas composition in the headspace of MAP fish may change significantly after packaging with a given initial gas mixture and gas/product ratio. The equilibrium gas composition is influenced by several factors e.g. the percentage of CO2 in the initial headspace gas (%CO2Initial), the initial gas/product volume ratio (G/P), temperature, pH and of corse permeability of the packing film. For flexible packaging films with low gas permeability the equilibrium concentration of CO2 in the headspace gas of MAP fresh fish can be predicted at different temperatures using eqn. 1-3 together (Ross and Dalgaard 2004). These equations take into account the effect of (i) the percentage of CO2 in the initial headspace gas (%CO2Initial), (ii) the initial gas/product volume ratio (G/P) and (iii) temperature. The equations does not take into account the effect of pH and results should not be considered highly accurate.
Eqn. 2 describes the temperature dependance of Henry's constant. In this equation, K is the absolute temperature (Carroll et al. 1991). These authors expressed KH as MPa/mole fraction and in eqn. 2, 101325 Pa/atm. and 2.4429 was used to convert this unit into mg CO2/liter of water or lipid/atm.
In Eqn. 3 dCO2 is the density of CO2 (1.976 g/l). |
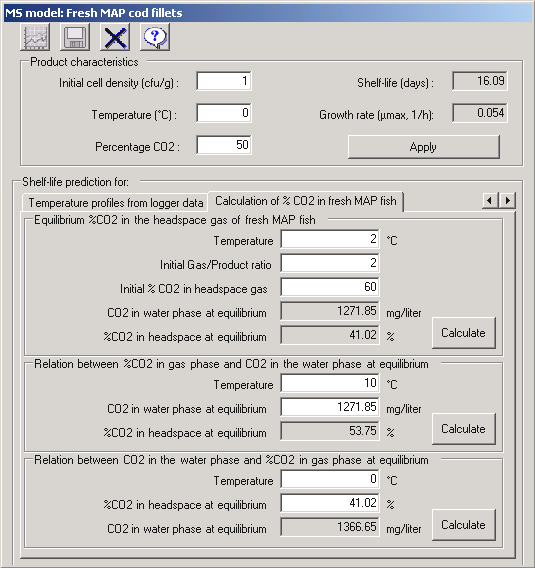
| To allow users of FSSP to conveniently calculate equilibrium concentrations of CO2 in MAP seafood the equations presented above have been included in a specific FSSP module 'CO2 equilibrium concentrations in MAP seafood'. As shown by the FSSP dialog box below, fresh cod fillets (~80 % water, 0% lipid) packed with an initial CO2 concentration of 60% and a gas/fish ratio of 2:1 (i.e. 200 ml gas and 100 ml water in the fish) at 2°C have an equilibrium concentration of close to 40% CO2 due to solubility of CO2 in the fish. With the same initial CO2 concentration but a gas/fish-water ratio of 4:1 the equilibrium concentration of CO2 will be close to 50% CO2. As also shown below the 'CO2 equilibrium concentrations in MAP seafood' module in FSSP allows users to calculate the relation between the equilibrium concentrations of dissolved and headspace CO2 at different temperatures. See Rotabakk et al. (2008) for more recent developments in the calculation of CO2 equilibrium concentrations in MAP seafood' |
 |