|
|
| Model | Listeria monocytogenes and lactic acid bacteria (LAB) in lightly preserved seafood including ready-to-eat products |
| References |
Mejlholm, O. and Dalgaard, P. (2007b). Modeling and predicting the growth of lactic acid bacteria in lightly preserved seafood and their inhibiting effect on Listeria monocytogenes J. Food Prot. 70 (11), 2485-2497.
Mejlholm, O.,
Bøknæs, N., Dalgaard, P. (2014).
Development and evaluation of a stochastic model for potential growth of
Listeria monocytogenes in naturally contaminated lightly preserved seafood.
Food Microbiol.
http://dx.doi.org/10.1016/j.fm.2014.06.006
Mejlholm, O., Dalgaard, P. (2015). Modelling the
simultaneous growth of
Listeria
monocytogenes and lactic acid bacteria in seafood and mayonnaise-based
seafood salads.
Food Microbiol.
http://dx.doi.org/10.1016/j.fm.2014.07.005
|
| Primary growth model | Logistic model with
delay and including interaction between
Listeria monocytogenes and LAB (Giménez
and Dalgaard, 2004) |
| Secondary growth model | Simplified cardinal parameter type model |
| Environmental parameters in model | Temperature,
atmosphere (CO2), water
phase salt/aw, pH, smoke components/phenol, nitrite and organic
acids in water phase of product (acetic acids, benzoic acid, citric acid, diacetate,
lactic acid and sorbic acids) |
| Product validation studies | Cold-smoked and
marinated (including 'gravad') salmon, Greenland halibut and trout (Mejlholm
& Dalgaard 2007b). Brined shrimp and mayonnaise-based seafood salads (Mejlholm and Dalgaard, 2015). Importantly,
this model has been shown to accurately predict the simuultaneous growth of
L. monoocytogens and lactic acid bacteria in naturally contaminated cold-smoked salmon and
naturally contaminated cold-smoked Greenland halibut (Mejlholm et al.
2014). |
| Range of applicability | Temperature (2-25°C),
atmosphere (0-100 % CO2), water phase salt (0.7-9.0 %), pH (5.6-7.7), smoke components/phenol (0-20 ppm),
nitrite (0-150 ppm in product), acetic acid (0-11000 ppm in water phase), benzoic acid (0-1800 ppm
in water phase),
citric acid (0-6500 ppm in water phase), diacetate (0-3000 ppm in water
phase), lactic acid (0-60000 ppm in water phase)
and sorbic acid (0-1300 in water phase). For mayonnaise-based seafood salads the model included in FSSP should not be used for products with pH below 6.0 (Mejlholm and Dalgaard, 2015). |
|
This model includes the effect of 12 environmental parameters (See Table above) on the simultaneous growth of L. monocytogenes and lactic acid bacteria (LAB) in lightly preserved seafood including many ready-to-eat products. Information on the lag time of L. monocytogenes in naturally contaminated lightly preserved seafood is still limited. Therefore, the growth model for L. monocytogenes can be used without lag time (fail safe predictions) or with lag time (more realistic predictions for naturally contaminated products). FSSP uses a relative lag time of 4.5 for L. monocytogenes. This lag time option is not available for LAB because numerous studies have confirmed these bacteria to grow without a significant lag time in lightly preserved seafood. See the FSSP dialog box and output window below (Fig. 1). FSSP predicts how high concentrations of LAB dampen and stops the growth of L. monocytogenes. This Jameson effect is most imporetant to accuratelty predict concentrations of L. monocytogenes and their influence on food safety. As shown in Fig. 1 and Fig. 2 the FSSP software allow these predictions to be carried out conveniently for constant and variable temperature storage conditions. |
|
|
|
Fig. 1. The graph above shows the predicted growth of L. monocytogenes and LAB for vacuum-packed cold-smoked salmon. Predictions are shown for two products with different smoke intensity (5 or 15 ppm phenol) and without or with added acetic acid. As shown FSSP predicts the time needed for the concentrations of L. monocytogenes and LAB to increase 100-fold under the selected product characteristics and storage conditions. The concentrations of L. monocytogenes and LAB shown in the bar at the bottom of the output window was obtained by using the mouse to click on the graph at a specific point in time. |
FSSP can predict growth of L. monocytogenes and LAB under changing temperature storage conditions. Simple temperature profiles can be typed in as 'Series of constant temperatures' whereas actual product temperature profiles most often are entered as 'Temperature profiles from data loggers' (Fig. 2).
|
|
|
Fig. 2. Effect of two simple temperature profiles on the predicted simultaneous growth of L. monocytogenes and LAB. |
|
|
|
|
Eqn. 1. Primary model for simultaneous growth of Listeria monocytogenes and LAB. tlag-Lm is the lag time for L. monocytogenes. Other model parameters are described in the text above the equation. |
|
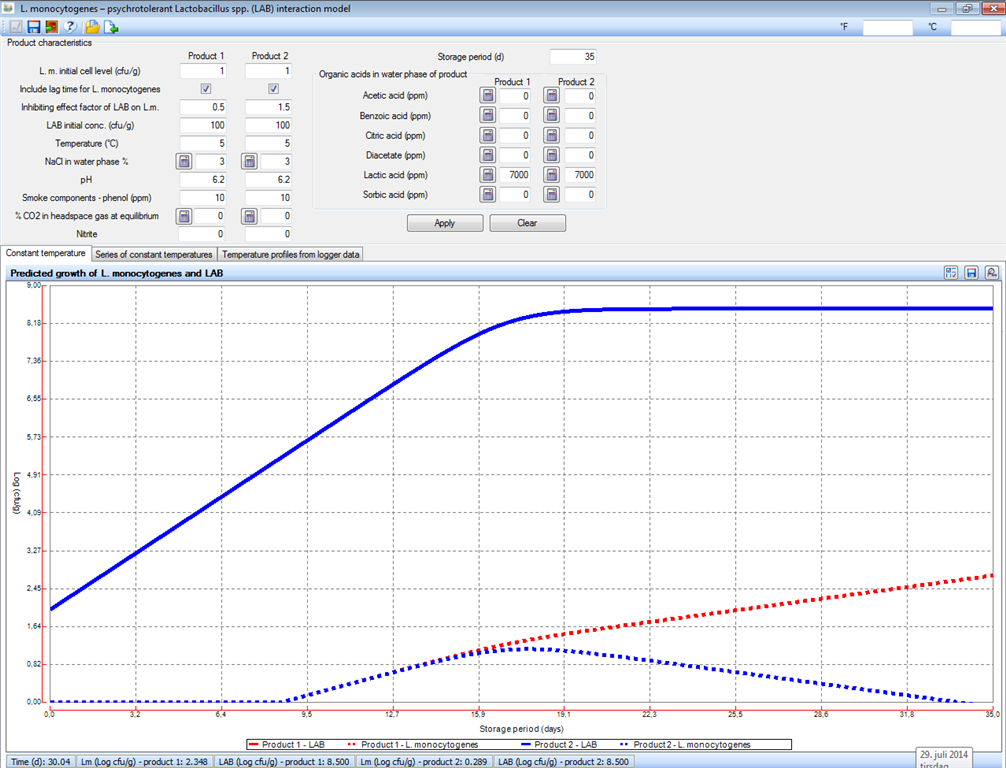
Fig. 3. Examples of how values of 0.5 and 1.5 for the competition factor (γ) influence the inhibiting effect of LAB on the predicted growth of L. monocytogenes. |
|
|
Listeria monocytogenes secondary model (Eqn. 2a)
|
Lactic acid bacteria secondary model (Eqn. 2b)
|
|
Eqn.
2. Secondary growth and growth boundary models for L. monocytogenes
and lactic acid bacteria
|
|
|
|
|